1) Welcome to the next installment of our key #oncology meeting highlights via #accredited #tweetorials from @onc_ce, your new (only) home for the latest education from #experts on #cancer care. #Physicians #nurses #pharmacists #PAs and #NPs all earn 0.5h CE/#CME by following!
2) Now we are covering @ASCO #GI22, which just wrapped up this past week –only the most current updates for OUR followers! Our expert author is Dr. Suneel Kamath (@SKamath_MD) from @ClevelandClinic in Ohio. The topics are gastro-esophageal cancers & HER2-targeted therapies.

3) These meeting summaries on @onc_ce and its companion website http://www.oncologytweetorials-ce.com are supported by an educational grant from Bayer and content is intended for #healthcare providers. Faculty disclosures are listed at http://www.oncologytweetorials-ce.com/disclosures/.
4) @ASCO #GI22 is a primary international scientific symposium for interaction and exchange among basic scientists and clinicians working in #GIcancer. @ASCO #GI22 was a hybrid meeting this year, with many renowned researchers welcoming the opportunity to collaborate FTF!
5) So let's start with a quick knowledge check. Which of the following HER2-targeted therapies are FDA-approved in HER2+ gastro-esophageal adenocarcinoma after progression on trastuzumab?
a. trastuzumab + pertuzumab
b. lapatinib
c. trastuzumab deruxtecan
d. tucatinib
6) For many years, nothing worked beyond 1st-line chemo + trastuzumab in HER2+ gastro-esophageal adenocarcinoma. Thankfully, that all changed.
7) Enter trastuzumab deruxtecan (a.k.a. #TDXd), a forerunner in a burgeoning group of antibody-drug conjugates (ADCs).
8) As a reminder, trastuzumab deruxtecan is an #ADC composed of an anti-HER2 (human epidermal growth factor receptor 2) antibody, a cleavable tetrapeptide-based linker, and a cytotoxic topoisomerase I inhibitor.
9) It was previously shown (🔓https://www.nejm.org/doi/full/10.1056/NEJMoa2004413) to have antitumor activity in a pretreated population of HER2+ gastric or gastroesophageal junction adenocarcinoma. In the #DESTINY-Gastric01 trial, T-DXd had better RR, PFS, & OS than physician’s choice of #chemotherapy.
10) Final overall survival data from DESTINY-Gastric01 were presented at @ASCO #GI22 by Kensei Yamaguchi, MD at The Cancer Institute Hospital of JFCR.
This was a fairly large, randomized study that was conducted entirely in Asia. The two study arms were well-balanced.

11) The waterfall plots for T-DXd vs. chemo are impressive. ORR was significantly better with T-DXd (42.0% vs. 12.5%), as was duration of response (12.5 vs. 3.9 months).

12) Now for the money shot. T-DXd significantly improved OS compared to physician’s choice chemotherapy, 12.5 months vs. 8.9 months, with a strong HR of 0.60.

13) T-DXd isn’t the easiest drug in terms of adverse events. Grade ≥ 3 AEs occurred in 85.6% of T-DXd patients vs. 56.5% with chemotherapy. Bone marrow suppression and GI toxicities are common.
14) Beware of #ILD/pneumonitis. It occurred in 12.8% of patients in DESTINY-Gastric01, including one grade 4 event and one treatment-related death from pneumonia.
15) Nevertheless, T-DXd received #FDA approval in January 2021 and is a preferred drug for 2L+ HER2+ gastro-esophageal adenocarcinoma in the @NCCN guidelines.
16) With the KEYNOTE 811 regimen of FOLFOX + trastuzumab + pembrolizumab becoming the new standard of care for HER2+ gastro-esophageal adenocarcinoma, you may be wondering if the DESTINY-Gastric01 results still hold up?
17a) An exploratory analysis of the trial (https://meetings.asco.org/abstracts-presentations/204790) presented by Kohei Shitara, MD at National Cancer Center Hospital East, Kashiwa, Japan says they do.
17b) In fact, it seems T-DXd works better after prior immune checkpoint inhibitors. And ILD rates were equivalent with or without prior immune #checkpoint inhibitors.

18) OK, let’s switch gears to one of the best stories in modern oncology, #immunotherapy in MSI-H cancer. Today, let’s focus on early-stage, MSI-H gastro-esophageal cancer.
19) We’ve known for years that locally-advanced MSI-H gastro-esophageal #adenocarcinomas have a better prognosis compared to MSS tumors and traditional fluoropyrimidine + platinum-based #chemotherapy has questionable benefit and may even cause harm in MSI-H tumors.
20) Maybe perioperative immunotherapy would be better? The #NEONIPIGA trial, presented by Thierry Andre, MD from Sorbonne University, Department of Medical Oncology, Saint-Antoine Hospital, AP-HP studied this exact question.
21) Here’s the study design. Note that low-dose #ipilimumab was used.

22) 32 patients were included. It is a small group, but fairly representative of what we see.

23) A whopping 59% of patients had pathologic complete responses and💯% of patients who went to surgery achieved R0 resections. And these survival curves are on 🔥!

24) Two patients did not go on to surgery because they had complete responses by endoscopy. Amazing. Only one patient had metastatic progression.
Importantly, only 25% of patients experienced grade 3+ TRAEs. The low dose of ipilimumab really helps here.
25) This was a great proof-of-concept study with earth-shattering results. The trial also raises the tantalizing question: Can we skip surgery entirely for those with #pCR? Time will tell.
26) It will also be exciting to see what #neoadjuvant #nivolumab + #ipilimumab can do in the #MSS population. This question is currently being investigated in a cooperative group study:
27) Let’s switch gears to the advanced/metastatic setting, where A LOT has changed in the last year.
28) And let's close today with a cliffhanger–a series of T/F statements. Please commit to true or false for each one and come back tomorrow to find out if you were right–and to grab that CE/CME! @YJanjigianMD @marklewismd @guptaarjun90 @LizzySmyth1 @KlempnerSam @drjasonstarr
29a) Regarding FDA approvals for frontline treatment in advanced/metastatic gastric and esophageal cancer, is it true that nivolumab + fluoropyrimidine and platinum-based chemotherapy is approved for adenocarcinoma, but only with PD-L1 CPS ≥ 5%?
29b) Regarding FDA approvals for frontline treatment in advanced/metastatic GE cancer, is it true that Pembrolizumab + fluoropyrimidine and platinum-based chemotherapy is approved for squamous cell carcinoma, esophageal and GEJ adenocarcinoma, but only with PD-L1 CPS ≥ 10%?
29c) Regarding FDA approvals for frontline treatment in advanced/metastatic gastric and esophageal cancer, is it true that nivolumab + fluoropyrimidine and platinum-based chemotherapy is approved for adenocarcinoma, regardless of PD-L1 CPS?
29d) Regarding FDA approvals for frontline treatment in advanced/metastatic GEl cancer, is it true that pembrolizumab + fluoropyrimidine and platinum-based chemotherapy is approved for squamous cell carcinoma, esophageal and GEJ adenocarcinoma, regardless of PD-L1 CPS.
30) Mark your answers and come back tomorrow!
31) Welcome back!! We are highlighting new data from @ASCO #GI22 on gastro-esophageal cancers & HER2-targeted therapies. Our expert author is @SKamath_MD from @ClevelandClinic. The topics are gastro-esophageal cancers & HER2-targeted therapies.
32) You are just a few🖱️clicks away from 🆓CE/#CME for #physicians #physicianassociate #nursepractitioners #nurse #pharmacist. Nods to @DrCrystalD @KoheiShitara @BiachiTiago @ILSONDavid @MaenAbdelrahim @dralanburguete @aakonc @pashtoonkasi @ShepardDale
33) Yesterday's barrage of T/F questions? Did you answer? If not scroll ⬆️and do so NOW. Hint: two are true and two are false.
34) So the correct answers: a & b are FALSE and c & d are true!
The current landscape for immunotherapy in gastro-esophageal cancers is complex. I summarized things in an earlier ASCO Daily News editorial (🔓https://dailynews.ascopubs.org/do/10.1200/ADN.21.200752/full/)
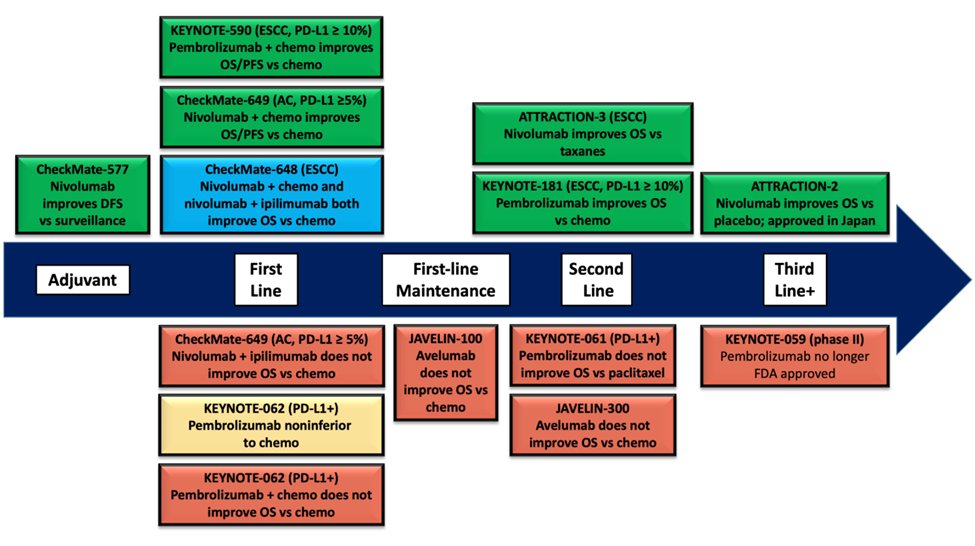
35) At @ASCO #GI22, we heard updated results from CheckMate 649 from Kohei Shitara, MD of the National Cancer Center Hospital East and updates on KEYNOTE-590 from Jean-Philippe Metges of the CHU Brest–Institut de Cancerologie et d’Hematologie ARPEGO Network.
36) As you recall, CheckMate 649 showed nivolumab + FOLFOX was superior to FOLFOX alone with improved PFS and OS. The updated survival curves with minimum 24 months of follow up look just as good.

37) The most useful thing we learned at @ASCO #GI22 were these subgroup analyses based on PD-L1 CPS.

38) PD-L1 CPS is by no means a perfect test, but it definitely appears that patients with PD-L1 CPS < 5% tumors gain little to no benefit with nivolumab + chemo vs. chemo alone (median OS: 12.4 vs. 12.3 months, HR: 0.94 with 95% CI clearly crossing 1).
39) And as we saw before, TRAEs were increased by 33% with nivolumab + chemo (60%) vs. chemo alone (45%).
40) For patients with PD-L1 CPS < 5% tumors, are 3 days of OS improvement worth a 33% increase in grade 3-4 AEs? You be the judge.
41) Let’s move on to the #KEYNOTE-590 updates from @ASCO #GI22. As you recall, this trial showed improved overall survival with pembrolizumab + chemo vs. chemo alone in esophageal squamous cell carcinoma (ESCC) and esophageal/GEJ adenocarcinoma.
42) Updated survival curves look just as good as the initial presentation.

43) The most important update from this trial is that the esophageal/GEJ adenocarcinoma subset (27%) still showed improved OS and PFS.

44) I’m confused about why KEYNOTE-590 was positive in the esophageal/GEJ adenocarcinoma subset. The earlier, much larger and still randomized KEYNOTE-062 did not show an OS benefit for pembro + chemo in the same population.
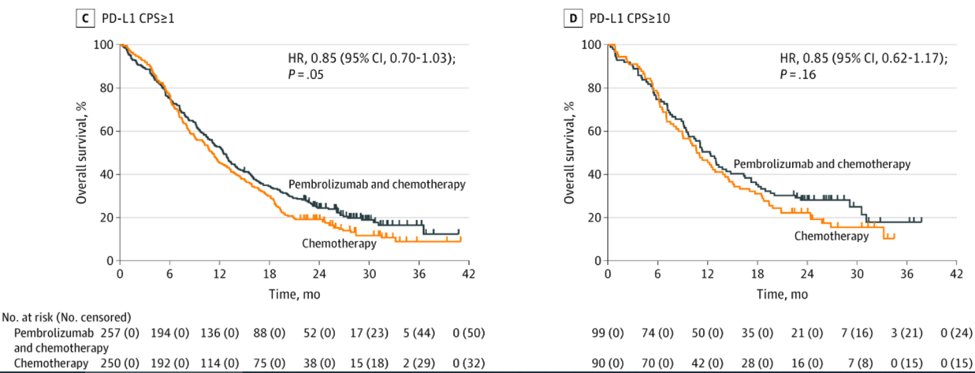
45) It was great to see that quality of life was preserved with chemo-immunotherapy compared to chemo alone. We know when we combine drugs that AEs go up, so very reassuring to see this didn’t hurt #QoL.

46) In summary, ESCC is easy. Chemo-immunotherapy with #pembrolizumab is a winner. Gastro-esophageal adenocarcinoma is less clear.
47) In the 🇺🇸, both nivolumab and pembrolizumab are #FDA-approved in combination with chemo for 1st-line patients regardless of PD-L1 CPS, but in 🇪🇺, the #EMA took a more narrow stance and approved nivolumab for PD-L1 CPS ≥ 5% and pembrolizumab for PD-L1 CPS ≥ 10%.
48) I like the @NCCN stance, where they give stronger category 1/2A recommendations for PD-L1 CPS ≥5% for nivolumab + chemo and for PD-L1 CPS ≥10% for pembrolizumab + chemo, and weaker category 2B recommendations for PD-L1 CPS below the respective cutoffs.

49) The reality is, the low PD-L1 CPS populations have no FDA-approved immunotherapy indications after 1st line. So if you ever want to go give IO, you have to do it up front. There may be a rare person who will get a long, durable response with IO despite low PD-L1 expression.
50) And that's it! Now go and claim your FREE CE/#CME at http://www.oncologytweetorials-ce.com/GE_HER2_ADC_IT and FOLLOW US for more #accredited #tweetorials for #physicians #pharmacists #nurses. Bravo to @ASCO #GI22 for keeping the show going!
Originally tweeted by @onc_ce (@onc_ce) on February 2, 2022.